Smart and Autonomous Medical Systems (SaAMS) Collaborative Community
SaAMS Collaborative Community
What are Smart and Autonomous Medical Systems (SaAMS)?
Smart and Autonomous Medical Systems (SaAMS) describe medical systems in which smart apps connect to medical devices to deliver transformative patient-care solutions safely and efficiently. SaAMS may use sophisticated algorithms that interact with interoperable medical devices to perform tasks that improve patient safety or efficiency, make decisions, automate processes, enhance vigilance, personalize patient and user experiences, advance healthcare equity, and solve historically intractable problems. These algorithms may be based on artificial intelligence (AI) to adapt to new information, make predictions, and operate autonomously.
Why are SaAMS Vital?
The demands of the COVID-19 public health emergency (PHE) provided one example of the need for SaAMS. The PHE drove a community of federal, industry and academic stakeholders to work together to rapidly advance autonomous medical device capabilities using intravenous infusion pumps and lung ventilators to enable hybrid virtual/in-person care models in diverse healthcare delivery environments. [1,2] Innovation initiatives such as these demonstrated that healthcare delivery can be transformed by applying automation technologies used in other domains. Solutions can range from lower levels of autonomy (such as adaptive cruise control, smart alarms, or guardrail dosing limits for intravenous medication pumps) to higher levels of autonomy (such as automated anesthesia). Integrating actuators, sensors, and smart clinical algorithms are the foundational components of these SaAMS. Solving long-standing gaps in clinical technology effectiveness and patient safety require SaAMS-based solutions.
Examples of SaAMS applications:
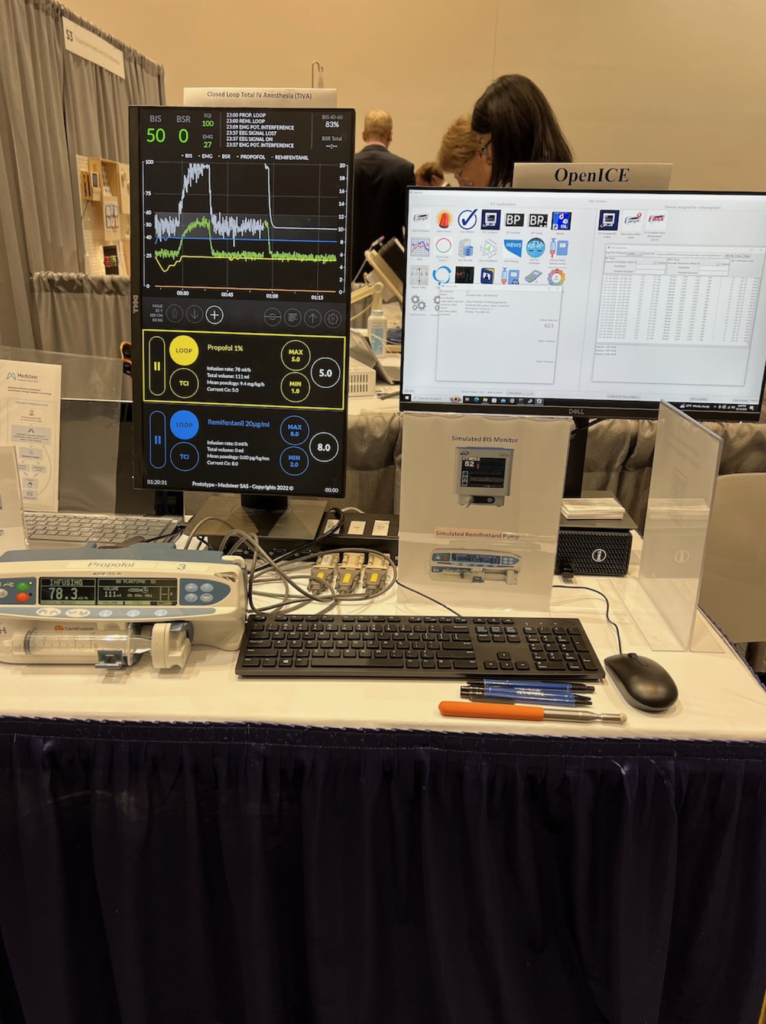
- Automated closed loop control of intravenous anesthesia (ACLIVA): Closed-loop intravenous sedation and anesthesia have wide-ranging clinical utility in intraoperative and ICU settings, and for military and civilian austere medical care. [3]
- Closed-loop blood pressure control: Research has shown that closed-loop vasopressor therapy in which vasopressor medication is infused to achieve a target arterial pressure range can minimize intraoperative hypotension and maintain SAP within 10% of the target range for >90% of the case time. [4]
- Closed-Loop IV fluid administration: “For fluid management and administration, the advantages of closed-loop technology are clear, especially in conditions that require precise care to improve outcomes, such as peri-operative care, trauma, and acute burn care.” [5]
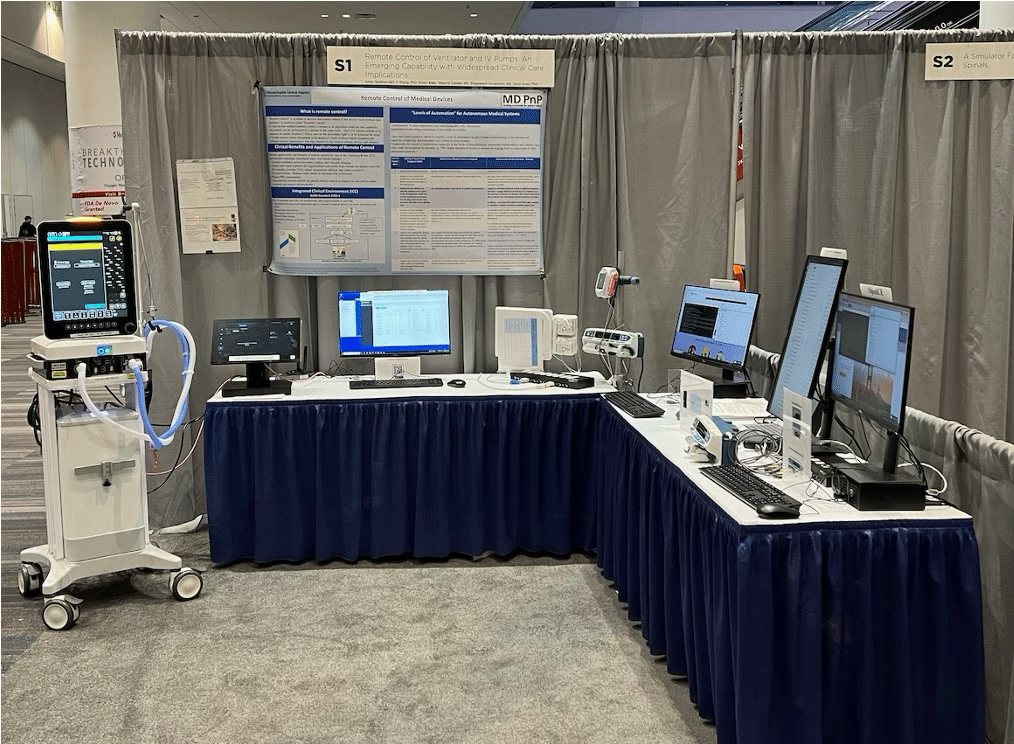
- Remote IV Infusion Pump Control and Remote Lung Ventilator Control: Remote control can facilitate care of patients undergoing anesthesia/sedation in hazardous environments (e.g. presence of ionizing radiation for neuroimaging), in settings where the IV pump or the ventilator cannot be readily accessed due to distance (e.g. certain OR and interventional procedures), and in airborne infection isolation rooms to protect caregivers from infection and enforce protective (reverse) isolation (e.g. large surface-area burns). [6]
- AI-based predictive clinical analytics:
Artificial intelligence (AI)‐based predictive analytics can provide real-time clinical analytics and dynamic visualization of patient anesthetic state, and “use machine learning and other modern statistical techniques to provide early warning of events of clinical deterioration such as sepsis, respiratory failure, [and] hemorrhage, and emergent intensive care unit (ICU) transfer.” [7]
Smart alarms that improve sensitivity to clinically significant events and enhance specificity to reduce non-actionable alarms and reduce alarm fatigue.
The Need for a SaAMS Collaborative Community:
A Collaborative Community can address challenging medical technology needs that no single manufacturer or other entity may be able to accomplish alone. This includes identifying and advancing key enabling device features and clinical system capabilities that address complex engineering and clinical challenges (e.g. external control of pumps, safe fallback modes of closed-loop systems). [8,9] We have formed a Collaborative Community, as described by the FDA, “to achieve common outcomes, solve shared challenges, and leverage collective opportunities” to advance the maturity, adoption, and clinical use of SaAMS to improve patient care. [10] The SaAMS CC is listed on the FDA website under “Collaborative Communities with CDRH Participation“.
Participants in the SaAMS Collaborative Community include a wide range of experts including manufacturers, clinicians, engineers, researchers, and the US FDA, to collaborate on the development of evidence to support safety. This safety framework is intended to provide precompetitive evidence for use in the regulatory process to de-risk commercial development and increase the safety, effectiveness, and clinical usability of these systems. It is anticipated that the framework will align with, and inform, applicable risk-management concepts, standards, and regulatory science.
Deliverables Under Consideration:
Deliverables are focused on evidence to support the safety and efficacy of SaAMS.
- Concept(s) of operation of the SaAMS systems including descriptions of clinical scenarios to assure conceptual interoperability
- Description of system components, architecture, and the necessary device data and control commands to assure safe operation (aligned with Medical Device Interface Data Sheets) [11]
- Use-case specific best practices
- Safety Assurance Case(s) and a shared risk model [12]
- Considerations for implementation in Integrated Clinical Environments (ICE) on platforms with interoperable, externally-controllable actuators, sensors, and smart clinical algorithms [13,14]
- Data logging / black box recorder requirements for quality assurance [15]
- Test methods – failure modes, fallback state(s), interoperability, communication degradation
- White papers, presentations, publications, content for inclusion in industry consensus standards
SaAMS Collaborative Community Sponsorship and Governance:
The SaAMS Collaborative Community is sponsored by the Medical Device Plug-and-Play Interoperability & Cybersecurity (“MD PnP”) research program and its Center for Smart and Autonomous Medical Systems (SaAMS) at the Massachusetts General Hospital Department of Anesthesia, Critical Care, and Pain Medicine, under the direction of Julian M. Goldman, MD. [14, 15] This is a pre-competitive initiative in which the parties agree to develop, gather, and analyze non-proprietary information to create publicly sharable evidence to support safe and effective SaAMS. Participating experts will be expected to develop and review documents produced by and for the group for technical excellence, completeness (topics are covered in sufficient depth), and comprehensiveness (not leaving out any major issues).
If you would like to join the SaAMS Collaborative Community, complete this form: https://bit.ly/SaAMS_Signup10
References:
- National Emergency Tele-Critical Care Network, https://mdpnp.mgh.harvard.edu/astra-portfolio/netccn/
- AAMI Consensus Report AAMI/CR511:2020 – Emergency Use Guidance for Remote Control of Medical Devices
- Closing the loop: automation in anesthesiology is coming. J Clin Monit Comput (2023). https://doi.org/10.1007/s10877-023-01077-3
- Systolic Arterial Pressure Control Using an Automated Closed-Loop System for Vasopressor Infusion during Intermediate-to-High-Risk Surgery: A Feasibility Study. J Pers Med. 2022 Sep 21;12(10):1554. doi: 10.3390/jpm12101554.
- Closed-Loop Controlled Fluid Administration Systems: A Comprehensive Scoping Review. J Pers Med. 2022 Jul; 12(7): 1168. Published online 2022 Jul 18. doi: 10.3390/jpm12071168
- Demonstration of Remote Control of Ventilators and Infusion Pumps to Support Disaster Care, US Army TATRC YouTube Channel, https://www.youtube.com/watch?v=XWAHkE73LrY, accessed November 28, 2023
- Beyond prediction: Off‐target uses of artificial intelligence‐based predictive analytics in a learning health system. `Learn Health Syst. 2023 Jan; 7(1): e10323. Published online 2022 Jun 23. doi: 10.1002/lrh2.10323
- IEC 60601-1-10:2007, Medical electrical equipment Part 1-10: General requirements for basic safety and essential performance, collateral standard: Requirements for the development of physiologic closed-loop controllers. (Last reviewed and confirmed 2020)
- Technical Considerations for Medical Devices with Physiologic Closed-Loop Control Technology (2023) https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-medical-devices-physiologic-closed-loop-control-technology
- Collaborative Communities: Addressing Health Care Challenges Together, https://www.fda.gov/about-fda/cdrh-strategic-priorities-and-updates/collaborative-communities-addressing-health-care-challenges-together
- Applying Medical Device Informatics to Enable Safe and Secure Interoperable Systems: Medical Device Interface Data Sheets, DOI: 10.1213/ANE.0000000000004251
- Introduction of Assurance Case Method and its Application in Regulatory Science (5/1/2019). https://www.fda.gov/media/125182/download
- Design Considerations and Pre-market Submission Recommendations for Interoperable Medical Devices (Final Guidance, 2017) https://www.fda.gov/media/95636/download
- ANSI/AAMI 2700-1:2019. American National Standard: Medical Devices and Medical Systems – Essential Safety And Performance Requirements For Equipment Comprising The Patient-Centric Integrated Clinical Environment (ICE) – Part 1: General Requirements And Conceptual Model.
- ANSI/AAMI 2700-2-1:2022 – Medical Devices and Medical Systems – Essential Safety and Performance Requirements for Equipment Comprising The Patient-Centric Integrated Clinical Environment (ICE): Part 2-1: Particular Requirements For Forensic Data Logging
- MGH MD PnP Program home page https://mdpnp.mgh.harvard.edu/