Our Portfolio
Projects + Themes
We perform a wide range of projects in support of our programmatic mission. This page briefly describes many of our projects and organizes them into “themes” to convey the synergistic relationship between our diverse technical, research, and educational activities.
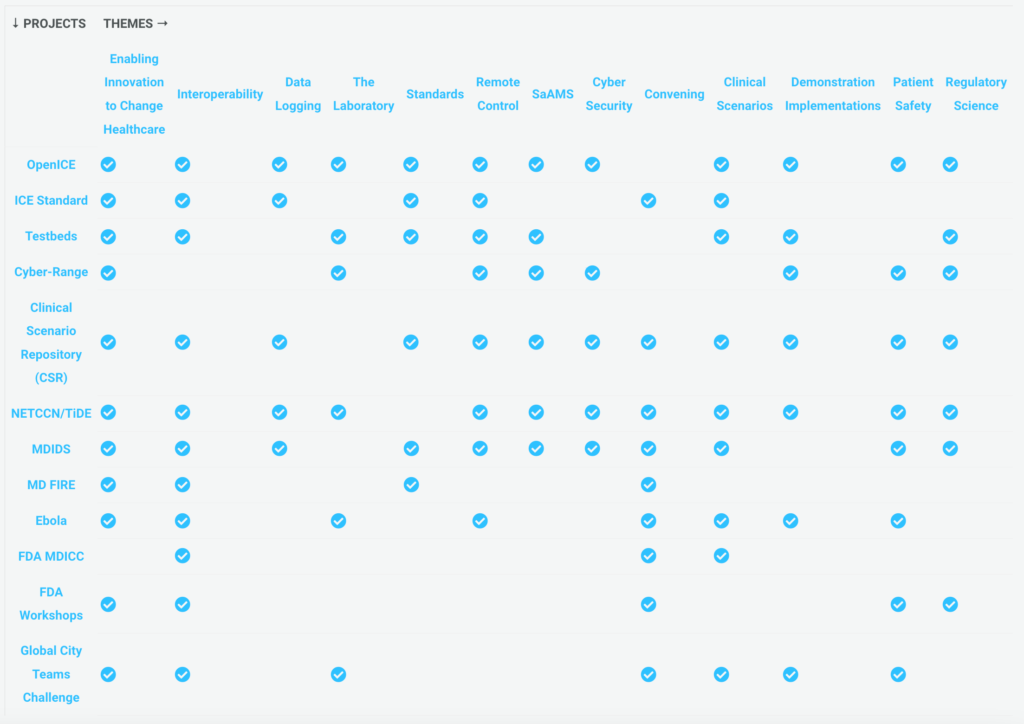
Project & Theme Matrix
This table lists Projects on the left side and Themes in columns. The matrix is being updated to be interactive.
[Note September 2023 – the entire website is being actively updated]
Theme Section
Themes
The following is a list of programmatic themes with brief descriptions.
Enabling Innovation to Change Healthcare
Innovation in healthcare has been impeded by a patient-care technology ecosystem that unlike smartphones, automobiles, and factories, has not been engineered for rapid improvement, high reliability, and safety. Our program develops and demonstrates solutions to reimagine healthcare delivery to improve patient safety and outcomes with an eye towards Smart and Autonomous Medical Systems (SaAMS).
Interoperability
Interoperability has been essential to the successful adoption of modern electronic technology. Would you buy a new printer, USB drive, smartphone, or digital camera if they were not interoperable with other devices, data file formats, computers, WifI or the Internet? Our research defines and demonstrates how to make medical equipment interoperable and the broad benefits that can be realized when they are integrated. As part of that analysis, we study how to achieve interoperability safely, by looking at interactions between devices that could lead to unsafe conditions.
Data Logging
Planes, trains, and automobiles have “black box recorders” that store comprehensive system-wide data that can be analyzed to determine the cause of an accident – and help prevent the next one. Our healthcare environments do not have a single, comprehensive, time-aligned, data log that can be used to analyze adverse events, identify a device or system failure, and use that data to prevent future negative outcomes. We have developed an open-source data logger, participated in the publication of the AAMI data logger standard 2700-2-1, and demonstrated how critical data can be captured in an Integrated Clinical Environment (ICE).
Patient Safety
“Getting Connected for Patient Safety” is the vision and tagline of our program. By transitioning vertically integrated, technologically fragmented, patient-care environments to “Integrated Clinical Environments”, solutions to improve patient care can be more rapidly developed, deployed, and improved.
Standards
Our team leads and participates in many medical device safety standards in standards development organizations including ANSI, AAMI, ISO, IEEE, IEC, UL, and ASTM. Often, our research findings are shared with standards development committees to help inform the development of published standards.
Remote Control
“If a television can have a remote control, why not a medical device?” – our program quoted by WCVB TV.
Adding remote control to a medical device can improve patient care by enabling more rapid responses to patient need by enabling changing the device’s setting from outside of the patient’s room. It can also enable multiple devices to be interconnected to build smarter, integrated clinical environments. We have been researching how to achieve safe remote control of medical devices.
Center for Smart and Autonomous Medical Systems (SaAMS)
Smart and Autonomous Medical Systems (SaAMS) include intelligent algorithms, safety interlocks, and closed-loop control systems. SaAMS describe the next generation of patient-care technologies enabled in part by deploying smart apps on platforms connected to interoperable medical and non-medical devices.
SaAMS enable the rapid development and deployment of innovative solutions and can reduce development costs by enabling re-use of existing platform resources and attached sensors and actuators (e.g. – monitors and therapy devices). The MD PnP program has established a Center for SaAMS within the MGH Dept. of Anesthesia, Critical Care, and Pain Medicine.
Testbeds
Our research and safety-engineering services require the use of specialized testbeds to develop and evaluate technologies ranging from remote medical device control, virtual medical care, closed-loop IV pump control, and networking technologies. We provide our testbeds and development experience as a service to interested parties.
At MHSRS 2023 we presented two posters that describe how we are testing remote control ventilators and automated closed-loop anesthesia technolgy.
Cybersecurity
Cybersecurity issues can impact the safety and operations of devices in many different ways. Our program has been engaged in diverse medical device and medical system cybersecurity projects. These include participating in FDA public workshops, serving as a funded performer in the Department of Homeland Security IMPACT program, and an FDA-MITRE project to define and prototype cyber-range capabilities in collaboration with commercial medical device manufacturers Philips and BD. Our program provides medical cybersecurity services to healthcare institutions and manufacturers.
MEHI
Our program is a member of the Mass Digital Health Sandbox Program which is administered by the Massachusetts eHealth Institute (MEHI). We provide lab services to innovative digital health companies through the Network.
Convening, Facilitation, and Advocacy
We have convened and hosted many conferences, scientific workshops, and standards development meetings. Our lab simulation and Virtual Hospital capabilities are often used for technology demonstrations and open-house educational events.
Clinical Scenarios
We use Clinical Scenarios to describes clinical situations in which improved technology, especially software apps connected to interoperable devices in the patient environment, could improve patient care. At the same time, we evaluate clinical scenarios to understand interactions that lead to unsafe conditions, assumptions, and constraints.
Demonstration Implementations
Much of our research is demonstrated through on-site and virtual events. Our lab’s diverse medical devices, patient simulators, and flexible networking infrastructure, supports diverse and complex demonstrations.
The Lab
Our 3200 sq. ft. lab suite, located in a MassGeneral Brigham research building in Central square in Cambridge, Mass, provides a clinical simulation environment with patient simulators, diverse medical equipment, pipeline gas supplies, and dedicated networking, to support clinical workflow, interoperability, device testing, and cybersecurity research.
The lab serves as a pre-clinical “Virtual Hospital” testbed to study and demonstrate future-focused technologies and clinical scenarios, and to showcase emerging Medical Internet of Things solutions.
Our program is a member of the Mass Digital Health Sandbox Program which is administered by the Massachusetts eHealth Institute (MEHI). We provide lab services to innovative digital health companies through the Network.
Regulatory Science
Our Regulatory Science research investigates and develops the tools, analysis methods, test beds, and standards necessary to evaluate and assure safe interoperable systems. We construct reference systems to evaluate interactions in Smart and Autonomous Systems (SaAMS). FDA/OSEL has a nice Regulatory Science description.
Projects
Our program is involved in a wide variety of research, education, and testing projects. These range from software development, cybersecurity analysis, standards writing, and real-time clinical technology simulation.
MDIDS (Medical Device Interface Data Sheets): We have been developing methods and reference compendium of medical device interface capabilities and data elements which could enable more complete, effective, and safe, device integration.
Clinical Scenario Repository (CSR): The CSR is a tool to elicit and document clinical situations in which improved technologies – especially those enabled through Integrated Clinical Environments – could improve patient safety and clinical care. We have used the CSR to capture “Good Ideas for Patient Safety” (GIPS) from clinicians and engineers.
OpenICE: OpenICE is an Open-source reference implementation of the AAMI Integrated Clinical Environment (ICE) Standard. The OpenICE software platform consists of software device adapters to connect medical devices (including anesthesia machines, ventilators, and patient monitors), OMG DDS standard-based middleware, and many demonstration applications. We use OpenICE as a prototyping environment and requirements-generating tool in many projects. It is freely downloadable from GitHub.
NETCCN/TiDE: Many geographic regions do not have ICU care available. Regions without ICU beds are not likely to have clinicians who are trained to use ventilators – even if the equipment becomes available in response to a regional or national emergency. A means to reliably and effectively support healthcare workers who deliver critical care has been demonstrated through this U.S. gov-supported research portfolio on tele-critical.
Intravenous (IV) Infusion Pump Testbed: IV pumps provide essential capabilities for a wide range of medical care, from routine fluid and medication delivery, to closed-loop control of anesthesia medications. In order to assure that current and future IV pump designs will support Smart and Autonomous (SaAMS) medical application, we developed an IV pump testbed to characterize and test the performance of infusion technologies, ranging from pump delivery accuracy to device interoperability.
Cyber-Range: Our lab contains integrated cyber-range capabilities to test and evaluate cybersecurity vulnerabilities and their mitigation – especially the impact on medical device system safety and performance. Components of the cyber-range include network manipulation and emulation tools, network traffic capture and analytics tools, and a highly configurable infrastructure including virtual networking capability.
More information is available on the lab Services page.
Return to table →
ICE Standard: Interdisciplinary standards-writing meetings convened by the MD PnP program from 2004–2008 identified key capabilities of a patient-centric Integrated Clinical Environment. The ICE standard Part I, describes the foundational architecture and requirements. It was published by ASTM in 2009 as F2761, and subsequently transferred to AAMI and published as ANSI/AAMI 2700-1, and recognized by the FDA.
FDA MDICC: The FDA formed a Medical Device Interoperability Coordinating Council (MDICC) to synchronize the multitude of efforts being pursued by different groups related to medical device interoperability. Our team led the Clinical Needs & Clinical Landscape group.
FDA Workshops: The Food and Drug Administration (FDA) Center for Devices and Radiological Health, in co-sponsorship with Continua Health Alliance and CIMIT/MD PnP, held a seminal public workshop entitled ‘‘Medical Device Interoperability: achieving safety and effectiveness.’’
Ebola: Our team was asked by US gov officials to rapidly identify medical technology-based solutions in support of Ebola care. Over a 20 day period, the MD PnP Program, with support by NIH/NIBIB, convened a group from government, academia and industry to prototype innovative approaches to improve patient care and reduce the risk of healthcare workers’ exposure to Ebola.
MD FIRE: “Medical Device Free Interoperability Requirements for the Enterprise” comprises a white paper and sample RFP and contracting language to promote the adoption of fully interoperable medical devices and systems in support of patient safety. MD FIRE was endorsed by the VA, Kaiser, Johns Hopkins Medicine, and Mass General Brigham.
SmartAmerica/Global City Teams Challenge: An initiative led by Presidential Innovation Fellows Sokwoo. Rhee and Geoff Mulligan. Our program convened multi-collaborator demonstrations in our lab.
Collaborate With Us
We invite new collaborators from all disciplines to join our community.
The MD PnP Program is involved with a wide variety of projects aimed at furthering the evaluation and adoption of open standards and technology for medical device interoperability and Open Health Platforms (OHP). Most of our portfolio has been funded through government grants and contracts, as well as industry engagements aligned with our mission.
The results of our research are typically released in the public domain through publications, demonstrations, and lectures.