MGH Dept of Anesthesia, Critical Care, and Pain Medicine
Center for Smart and Autonomous Medical Systems (SaAMS)
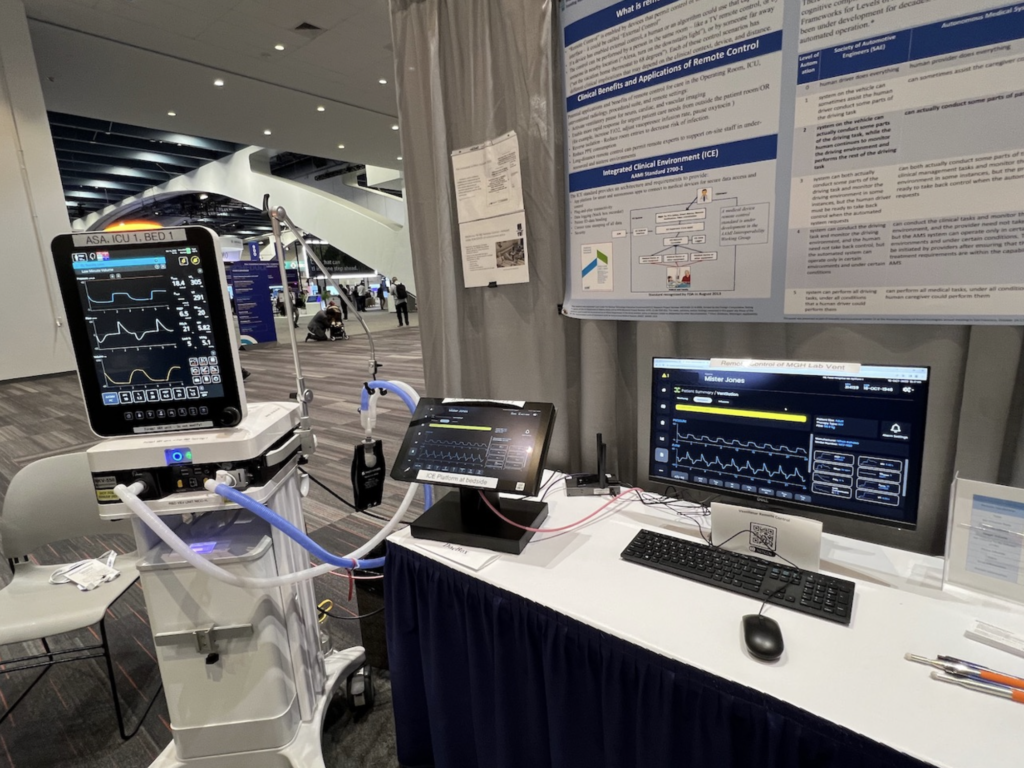
Smart and Autonomous Medical Systems (SaAMS) describe medical systems in which smart apps connect to medical devices to safely and efficiently deliver transformative patient-care solutions. SaAMS may utilize sophisticated algorithms that interact with interoperable medical devices to perform tasks that improve patient safety or efficiency, make decisions, automate processes, enhance vigilance, personalize patient and user experiences, advance healthcare equity, and solve historically intractable problems. They may utilize some form of artificial intelligence (AI), adapt to new information, make predictions, and operate autonomously.
The Center for SaAMS is dedicated to advancing SaAMS through a unified approach to research and development, including simulation, teaching, testing, policy, and lab services. It leverages our extensive virtual hospital testbed laboratories. The Center for SaAMS created the SaAMS Collaborative Community to develop and share pre-competitive evidence of safety and performance for SaAMS. More information about the Collaborative Community can be found here.
Key considerations for SaAMS include:
- Medical Device Informatics*
- Interoperability (Syntactic, Semantic, Technical, Conceptual) and Integration
- Integration architectures, such as the Integrated Clinical Environment (ICE) standard
- Real-world medical device performance
- Cybersecurity
- Clinical systems engineering
- Safety engineering
- Emergent behaviors
- Application and development of consensus medical device and health informatics standards
- App-development strategies and re-use
- Remote monitoring and control
- Pre-clinical Testing
- System forensic analysis methods
- Clinical scenario capture and analysis
- Management and maintenance strategies
*Applying the principles of medical device informatics is essential to achieve safe and effective SaAMS. Medical device informatics, a subset of biomedical informatics, deals with the study, design, and management of many facets of physical (hardware) and software medical device signal acquisition, data communication and analysis, device and interface performance characteristics, and component interactions. For example, identifying the essential information to exchange between components of the system (aligned with Medical Device Interface Data Sheets), is necessary for SaAMS. [1]
Reference:
[1] Applying Medical Device Informatics to Enable Safe and Secure Interoperable Systems: Medical Device Interface Data Sheets, DOI: 10.1213/ANE.0000000000004251