ICE Standard: Integrated Clinical Environment
Date: 2004–Present
Collaborators: ASTM, AAMI
Scope and Purpose of ICE
Interdisciplinary meetings convened by the MD PnP program from 2004–2008 (described on this web site) identified key capabilities of a patient-centric integrated clinical environment. These capabilities, such as comprehensive data acquisition for the EMR and the integration of devices to enable real-time decision support, safety interlocks, and closed-loop control, can be achieved through the functions described in the standard for the “Patient-Centric Integrated Clinical Environment” (ICE). The ICE standard was published in 2009 by ASTM as F2761-09. Subsequently, the standard was transferred to AAMI, and is now published as AAMI 2700-1.
The ICE late-stage committee draft may be downloaded here.
Click here to download a figure of ICE-compliant architecture, redrawn with detail added, from ASTM F2761.
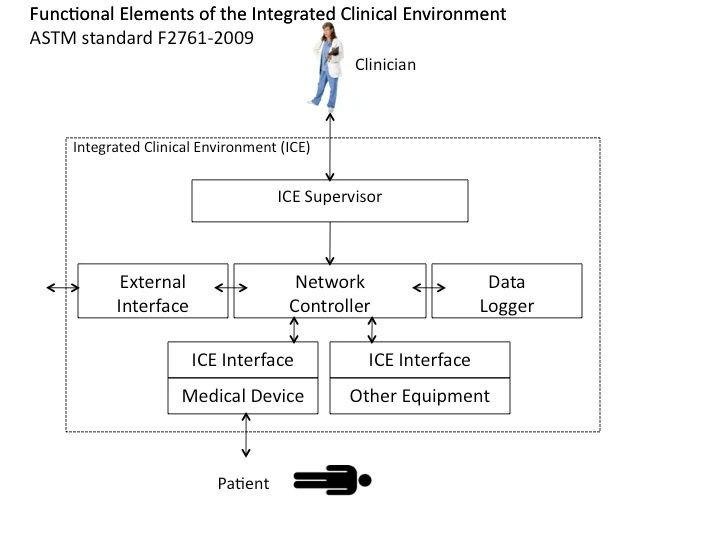
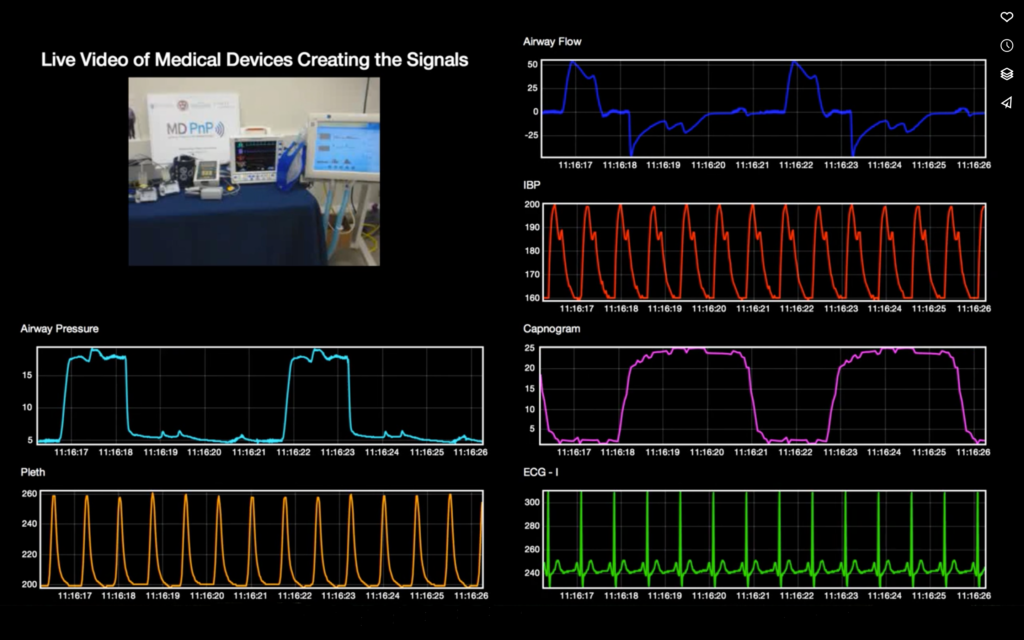
Background and Status of ICE Standardization Process
- The ICE standardization activity was initiated in 2006 by ASTM International, committee F29, based on foundational work performed by the MD PnP program and diverse collaborators from 2004-2006.
- The standard was developed under the auspices of ASTM Committee F29 Subcommittee F29.21, “Devices in the Integrated Clinical Environment,” chaired by Dr. Julian Goldman.
- The “ICE standard” – “Essential safety requirements for equipment comprising the patient-centric integrated clinical environment (ICE) — Part 1: General requirements and conceptual model” was published as F2761-09 in 2019 and re approved in 2013. The standard is numbered F2761-09(13) to reflect these dates. It can be purchased from ASTM International.
- The entire ASTM F29 standards portfolio has been transitioned from ASTM to Association for the Advancement of Medical Instrumentation (AAMI), as US Standards Development Organization. The next part of the ICE standard, the ICE Forensic Data Logger (or “black box recorder”) began development in 2016 within the AAMI SM-03 Interoperability Working Group (IOWG), co-chaired by Julian Goldman, MD, and Sandy Weininger, PhD.
- For AAMI IOWG committee information, contact Wil Vargus, AAMI Standards Director.
- The ICE standard (F2761) has become a foundational standard for new initiatives that benefit from the ICE architecture and clinical use cases (contained in F2761 Annex B). Related standards work includes AAMI-UL 2800 and AAMI SW95 ICE Data Logger.
ICE Data Logger
Medical devices are increasingly being networked, for example to populate the electronic health record (EHR). Problems with individual devices in such a system, or unexpected interactions between the devices, can cause device failures and may compromise patient safety. For example, in our lab we have documented a case of ventilator failure triggered by requesting data to send to the EHR. When this kind of incident occurs, clinicians, manufacturers, and regulators have the responsibility to investigate the cause of the problem and try to ensure that it does not happen again.
Planes, trains, and automobiles have “black box recorders” – or “data loggers” to support forensic data analysis in safety critical systems. We believe that this capability is essential for clinical environments as well. As described in standard ASTM F2761-09 on the Integrated Clinical Environment (ICE), even-logging functionality is necessary to address regulatory and liability concerns regarding networked medical device systems, and will also improve the forensic analysis of clinical adverse events and near misses.
When data from medical equipment is available in a suitable format for forensic data logging, the data can also be used to create novel data presentation displays, be provided to real-time or post-hoc analytics engines, and other applications to support clinical care.
We are implementing a basic open-source ICE Data Logger that will capture device and use data intended to facilitate analysis of adverse events and enable other types of analysis of device networks, and we plan to develop a functional prototype Data Logger that will capture data from medical devices in a time-synchronized, standardized, and trustworthy manner. This work will preview the power of these emerging capabilities, as existing standards do not yet support all the data that is needed from devices for recording and later analysis.
A prototype ICE Data Logger implemented by NIST based on OpenICE was presented at the Smart America Expo June 2014 in the Closed Loop Healthcare team.
A standard for ICE Data logging was published by as ANSI/AAMI 2700-2-1:2022 Medical Devices And Medical Systems – Essential Safety And Performance Requirements For Equipment Comprising The Patient-Centric Integrated Clinical Environment (ICE): Part 2-1: Particular Requirements For Forensic Data Logging
Description if the standard:
“ANSI/AAMI 2700-2-1 is part of the AAMI 2700 family of standards to achieve safe integrated clinical environments (ICE) (ANSI/AAMI 2700-1). It is intended for use by medical device and platform manufacturers and system integrators. It provides requirements for the recording, storage, and playback of data to support safety, quality assurance, and forensic analysis for medical devices, applications, and platforms. This document supports safe and secure device interoperability by providing general functional, performance, security, and interoperability requirements of ICE data logging systems.”
Historical note:
The online proceedings (PDF version) (Archived version) of the Global Harmonization Task Force 11 (GHTF 11, Washington, DC, October 2007) (Archived version) (PDF version) describe the recommendations of GHTF 10 (Lubeck, Germany, June 2006) to support the ICE standard.