Improving healthcare safety and accelerating innovation
MD PnP — the Medical Device "Plug-and-Play" Interoperability & Cybersecurity Program
The Challenge:
How can we make better use of medical technology to improve the safety of health care?
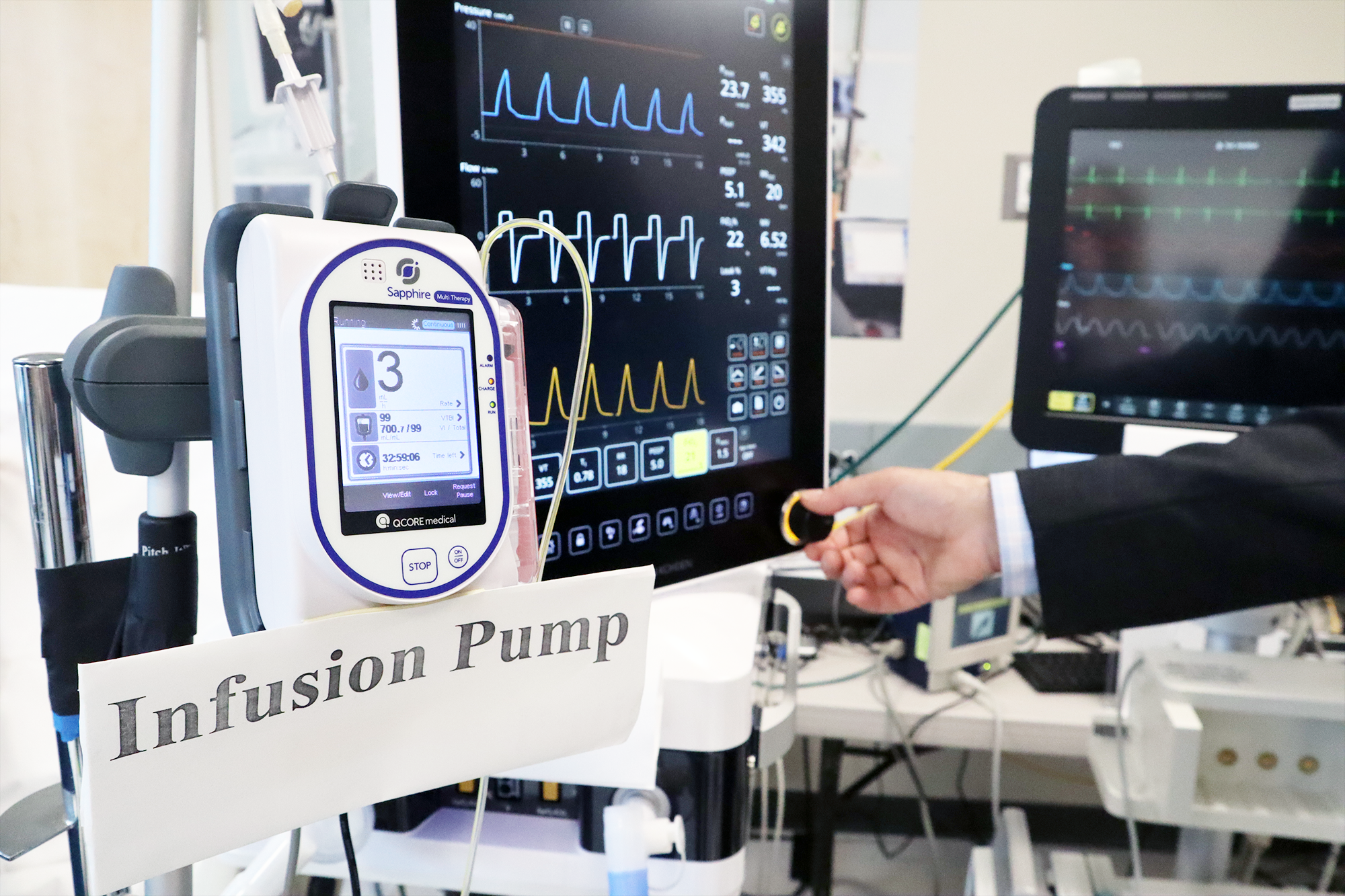
Medical devices are essential to the practice of modern medicine. Clinical measurements such as blood pressure and temperature, x-ray and ultrasound imaging, administration of intravenous medications, and support of critical life functions all require medical devices. However, despite our reliance on sophisticated medical equipment, most devices are not designed to interconnect with other devices and are not inherently secure. Therefore, it is difficult to integrated individual devices to create “Smart and Autonomous Medical Systems” (SaAMS) to improve patient care, prevent unnecessary accidents, and personalize care delivery.
The Answer
Collaborating on Smart and Autonomous Medical Systems (SaAMS)
Since its establishment in 2004 at the Massachusetts General Hospital, the Medical Device “Plug-and-Play” (MD PnP) Interoperability & Cybersecurity Program has been enabling the adoption of medical device interoperability as a foundation for the development of innovative medical “apps” that could communicate with interoperable medical devices on open-health platforms to improve patient safety, diagnosis, treatment, and research. Our program has contributed to international standards, published research, developed open-source software, and hosted collaborative lab demonstrations in support of this mission.
The “plug-and-play interoperability” envisioned when we founded the MD PnP Program in 2004 was intended to enable the development of Smart and Autonomous Medical Systems (SaAMS). Much has changed in the last 20 years, but the need to improve quality, safety, and accessibility of healthcare has only increased. The good news is that advances in computer and medical device technology are accelerating the development and adoption of safe and secure SaAMS.
Learn more about our Center for SaAMS and new SaAMS Collaborative Community.
"Integrated Clinical Environments (ICE) to Improve Safety and Enable Rapid Innovation"
Speed Diagnosis With Integrated Data
How Integrated Data Can Speed Diagnosis and Treatment of Monitored Cardiac Arrest
Featured Project
OpenICE
Examples of What We Do
Areas of Focus

Enabling innovation to change healthcare
Interoperability

Patient safety
Standards
Remote Control
SaAms

Cyber Security
The Lab
MEHI (Lab Testing Services)

Convening
Clinical Scenarios
Demonstration Implementations
Data Logging
The Latest
News + Events
Collaborators Past & Present










